May 1, 2023
How can EMA speed up access to cancer treatment for patients?

By Ehab Naim
When you reflect on the European Union (EU), you may think of its positions on freedom, public protection, or any of the other policies that have helped to make it a robust global leader. However, when it comes to life-saving treatments, there is still much work yet to be done. Let’s explore why.
The European Medicines Agency (EMA), the EU’s body responsible for evaluating the safety and effectiveness of pharmaceutical products, is an internationally-recognized regulatory authority. Despite its credible reputation, bureaucratic procedures still can hamper the agency. This is evident in the delays in introducing life-saving treatments. To better understand how and why, we can compare it to another well-established authority that advocates for better human health and shares values similar to that of the EMA: The United States Food and Drug Administration (FDA).
Approval timelines: Why is the FDA more efficient than the EMA?
A simple query on PubMed, a search engine that accesses the MEDLINE database of references and abstracts on life sciences and biomedical matters, about the differences between the FDA and EMA approval processes and timelines will yield a breadth of results. A significant body of the literature discusses the delays experienced by the EMA in approving cancer treatments. Despite both agencies having programs that presumably enable a faster introduction of drugs and treatments to the market, the FDA does it much faster.
The main drivers behind the faster approvals experienced with the FDA compared to the EMA are the shorter review times upon application submission. In addition, the FDA provides more regulatory options that allow faster product review, assessment, and patient access.
Evidence from a recent JAMA study
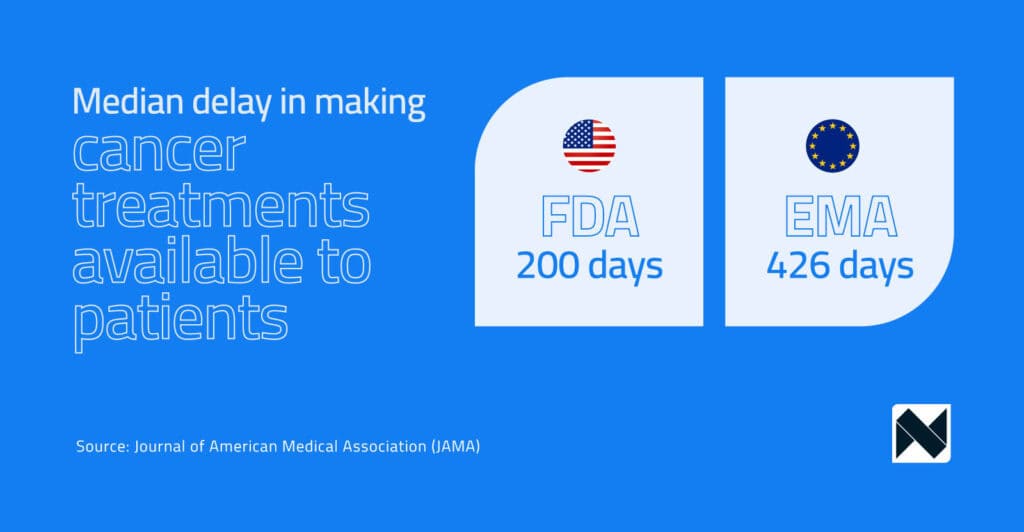
A recent study published in the Journal of American Medical Association (JAMA), which examined a decade’s worth of approval timelines (2010-2019) involving 89 oncology treatments, found that the FDA approved 95% of these therapies first If you think this is significant, then you should see the timelines…
In the study by Lythgoe et al., timelines between the two agencies varied significantly. Compared to the FDA , the median delay experienced by the EMA in making cancer treatments available to patients was over 240 days. To put things into perspective, the FDA took a median of 200 days to review an application compared to EMA’s 426 days. When we talk about cancer, every day matters!
The question is, was this the case prior to the results of the recent study? The answer is, unfortunately, yes. Another, older study by Roberts and colleagues examining the timelines for the market authorizations of cancer treatments between 2003 and 2010 had similar findings. In this study, delays experienced due to EMA processing timelines were 238 days more than that of the FDA!
Examining both studies shows that despite the extended timeframe of over a decade (2003 to 2019) in exploring oncology treatment approval timelines, little change has occurred.
EMA bureaucratic procedures delay cancer treatment access for European patients
Can the process at the EMA be faster? We at Narrativa think so!
A possible solution to expedite an application is to submit it earlier. One such way of doing this is through faster authoring of clinical study reports (CSRs) delivered to the EMA.
Patient narratives and Tables, Lists, and Figures (TLFs) are parts of a CSR that require investing a significant amount of time to produce. Automating these processes could translate into faster approvals. This is because automation using natural language processing (NLP) and natural language generation (NLG) could cut submission timelines from several months to weeks. This propagates a potentially faster drug introduction to the market and overcomes some of the delays experienced during EMA submissions.
Luckily, this is exactly where Narrativa specializes. We’ve worked for years to develop and perfect an artificial intelligence (AI) automation solution for life sciences that addresses the aforementioned problems quickly, accurately, and securely. Though we can’t control the pace at which regulatory bodies such as the EMA move, we can better empower businesses of all sizes to submit applications faster through the use of AI. Book a demo now to find out just how much time and money we can save you!.
About Narrativa
Narrativa is an internationally recognized content services company that uses its proprietary artificial intelligence and machine learning platforms to build and deploy digital content solutions for enterprises. Its technology suite, consisting of data extraction, data analysis, natural language processing (NLP) and natural language generation (NLG) tools, all seamlessly work together to power a lineup of smart content creation, automated business intelligence reporting and process optimization products for a variety of industries.
Contact us to learn more about our solutions!
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]Share







