June 6, 2023
Regulatory documentation automation: Get MORE of your day back!

By Ehab Naim
The use of artificial intelligence (AI) is gaining significant momentum in various healthcare fields. It has been implemented in drug research and discovery processes, analyzing medical images to correlate conditions and other areas. With respect to regulatory submissions, AI presents many opportunities, such as automating documentation like clinical study reports (CSRs). But can it do more? Let’s find out together!
First things first: Automating regulatory documents
Automating regulatory documents is no easy feat. It involves analyzing and connecting millions of data points to produce documents, like patient safety narratives. This requires the AI to not only understand the information included in these databases but also to reason. Why? The generated text that populates the CSR must be both logical and understandable to the reader. Technologies, such as generative AI, help with sequencing a cogent flow of information.
In addition to the above, Narrativa’s solutions can also do heavier lifting to create tables, lists, and figures (TLFs). Why is this important? Because in order to have a reliable outcome, like a patient narrative, the data fed to AI models need to be structured and organized for the AI to produce neatly formatted and correct TLFs in minimal time.
Why automate?
The benefit associated with automating regulatory documents is, first and foremost, the amount of time saved. AI models will go through numerous data points across multiple databases to compile the outcome in a fraction of the time it takes a human. It also adds a layer of quality checks that were not previously possible via conventional means due to the sheer size of the databases and how long it would take to validate every piece of information. This additional level of assurance could flag potential inconsistencies within databases so that proper measures are taken to address them or their potential impact on the result.
Additional benefits include more complete and accurate documents constructed from these databases since all inconsistencies are addressed before their submission to regulatory authorities. This significantly minimizes errors that could result in delays in introducing life-saving medications and treatments to the market.
Furthermore, it leads to tracing possible errors that occur during data entry so that such problems are addressed in subsequent studies during the data collection stage and before the database lock. This is significant, as it positively reflects on all subsequent downstream processes.
Narrativa’s solutions can detect and flag database inconsistencies during the document generation phase. This way, the quality of the authored documents and other outcomes are ensured because medical writers and other stakeholders involved in data collection, documentation, authoring, and submission are aware of the shortcomings associated with a certain database or patient. Let’s see an example.
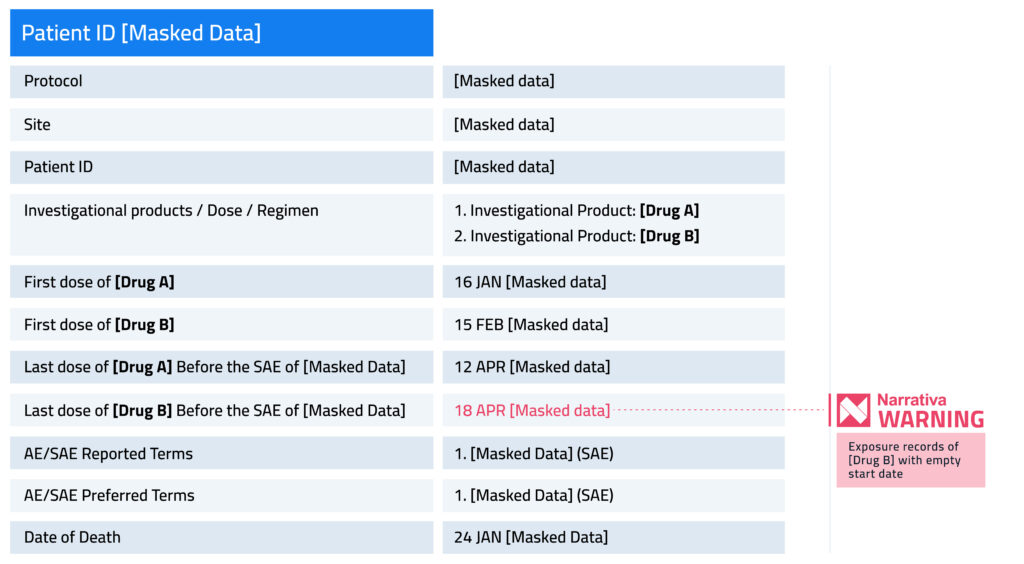
Masked data refer to confidential information; drugs A and B are masked information. Please note that the template is customized per the client’s request.
Less is more: Fewer errors lead to more time saved
Narrativa’s solution flags errors in databases by identifying the mandatory key information that is missing (e.g. an exposure date entry) and highlighting it to bring it to your attention. Many of our clients come to think of this as having a second set of very detail-oriented eyes.
Narrativa also detects weak or missing data links across different domains. That is to say that absent data may supply the exact kind of context that is critical to successfully generate outcomes. An example of this would be a missing record of a laboratory result tied to an adverse event. In this case, we would have a record of an adverse event but not any related information, like the associated lab results.
Finally, Narrativa has a suite of other solutions that ensure consistency and save time. An example of these tools is the anonymization and redaction solution, which scans thousands of pages of clinical documents within a few minutes to protect the privacy of trial participants.
About Narrativa
Narrativa is an internationally recognized B2B content services company that believes people and artificial intelligence are better together. Through its proprietary generative AI content automation platform, teams of all types and sizes are empowered to build and deploy smart composition, business intelligence reporting, and process optimization content solutions for internal and external audiences alike. Its tech stack, consisting of data extraction, data analysis, natural language processing (NLP) and natural language generation (NLG) tools, all seamlessly work together to produce content quickly and at scale. In this way, Narrativa supports the growth of businesses across a variety of industries, while also saving them both time and money. Accelerate the potential with Narrativa.
Contact us to learn more about our solutions!
Share







