Accelerate Regulatory Submissions
Automation of Clinical Study Reports (CSRs)
Narrativa Knowledge Graph® and Generative AI automate the generation of clinical study reports through data extraction and augmentation.
Solution trusted by



Up to a 6 week
Reduction in authoring time
50%
Reduction in reviews required
30 seconds
To generate the first draft
Clinical Study Reports Automation
Benefits of Automating CSR Generation
- Enhanced Efficiency: Streamlines processes and reduces authoring time, leading to greater operational efficiencies.
- Accelerated Approvals: Speeds up the regulatory submission and approval process.
- Cost Reduction: Significantly lowers costs and overhead associated with CSR creation.
- Fewer Reviews: Reduces the need for multiple review cycles, minimizing effort and errors.
- Improved Compliance: Ensures better regulatory adherence and alignment with industry guidelines.
Technology Powering CSR Automation
- Knowledge Graphs: Organizing and linking complex data for deeper insights.
- Generative AI: Crafting coherent and compliant narratives.
- Entity Extraction: Identifying and extracting key medical and regulatory entities.
- Data Clustering: Grouping relevant data points for structured reporting.
- Natural Language Processing (NLP): Transforming clinical data into readable and meaningful text.
Automation of Clinical Study Reports Using AI to Accelerate Regulatory Submissions
Narrativa’s Answer to Regulatory Affairs Automation
Narrativa harnesses Generative AI to transform clinical trial data into comprehensive CSRs. AI’s capabilities extend beyond text generation to include information extraction, question answering, etc. This technological advancement is set to revolutionize clinical trial design and reporting.
Automation of Clinical Report Writing
Automating CSRs enables pharmaceutical companies to streamline workflows, minimize costs, and ensure guideline adherence. By reducing human errors and review cycles, AI-driven CSR automation enhances efficiency. Key applications include automating Tables, Listings, and Figures (TLFs) and patient safety narratives, significantly reducing the workload for medical writing teams.
How Does Our Platform Work?
Narrativa’s platform automates CSR writing by extracting data directly from Tables, Listings, and Figures (TLFs) and ADaM (Analysis Data Model) structured datasets, converting them into structured prose. Instead of manually analyzing complex tables, our AI-powered system processes TLFs from various formats (PDF, Word, etc.), imports the data into Narrativa’s Knowledge Graph, and generates clear, concise narrative text—saving time and improving accuracy.
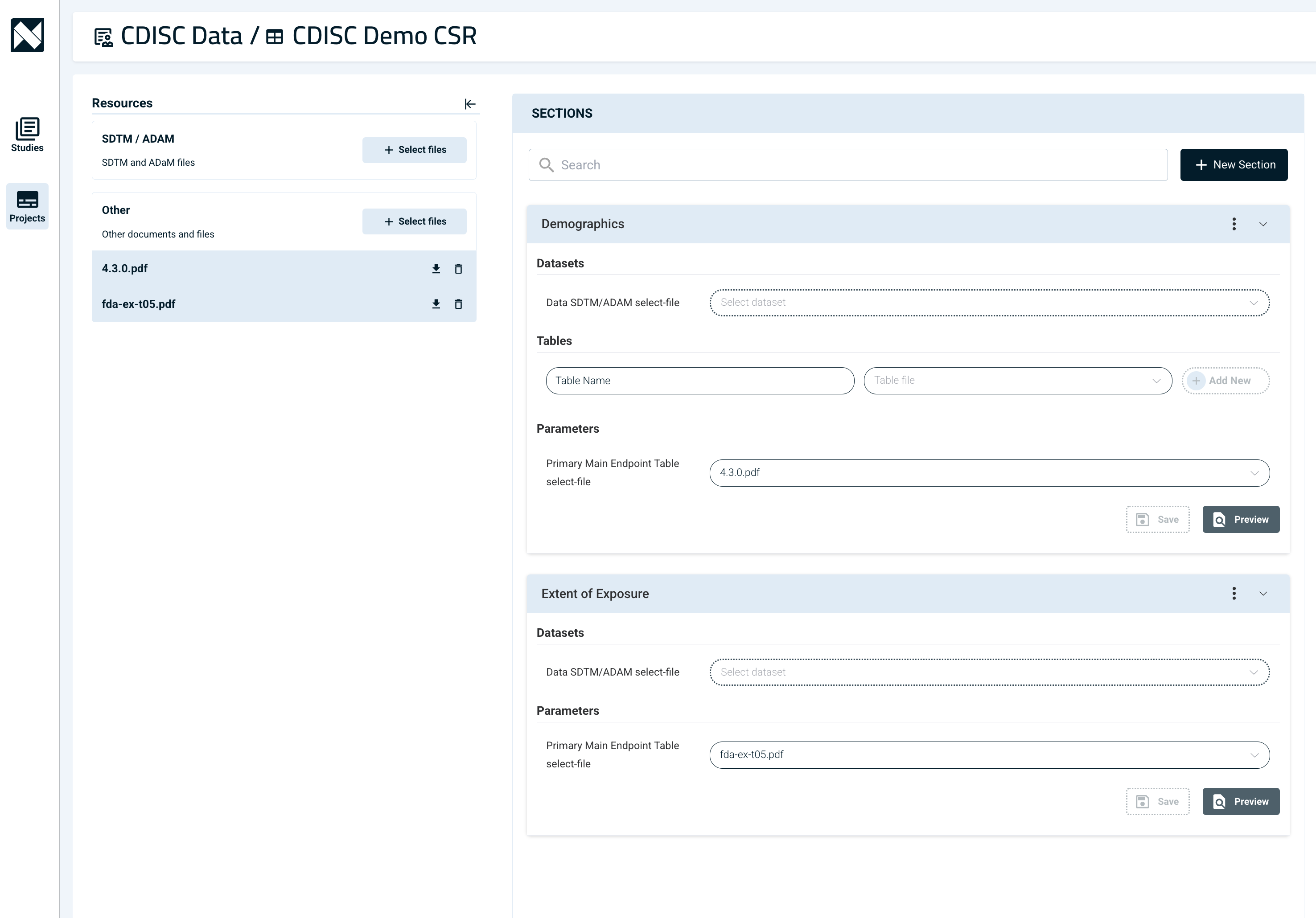
Our data-to-text AI models allow both system-generated and custom user prompts for flexible content generation.
Traceability and Validation
Since the platform generates an initial draft, the medical writer must review and validate the text for accuracy. To facilitate this, the system allows users to click on any data point, instantly locating the corresponding value in the original table or dataset.
This feature streamlines the quality assurance (QA) process, enabling quick verification and edits while keeping full control in the hands of the medical writer.
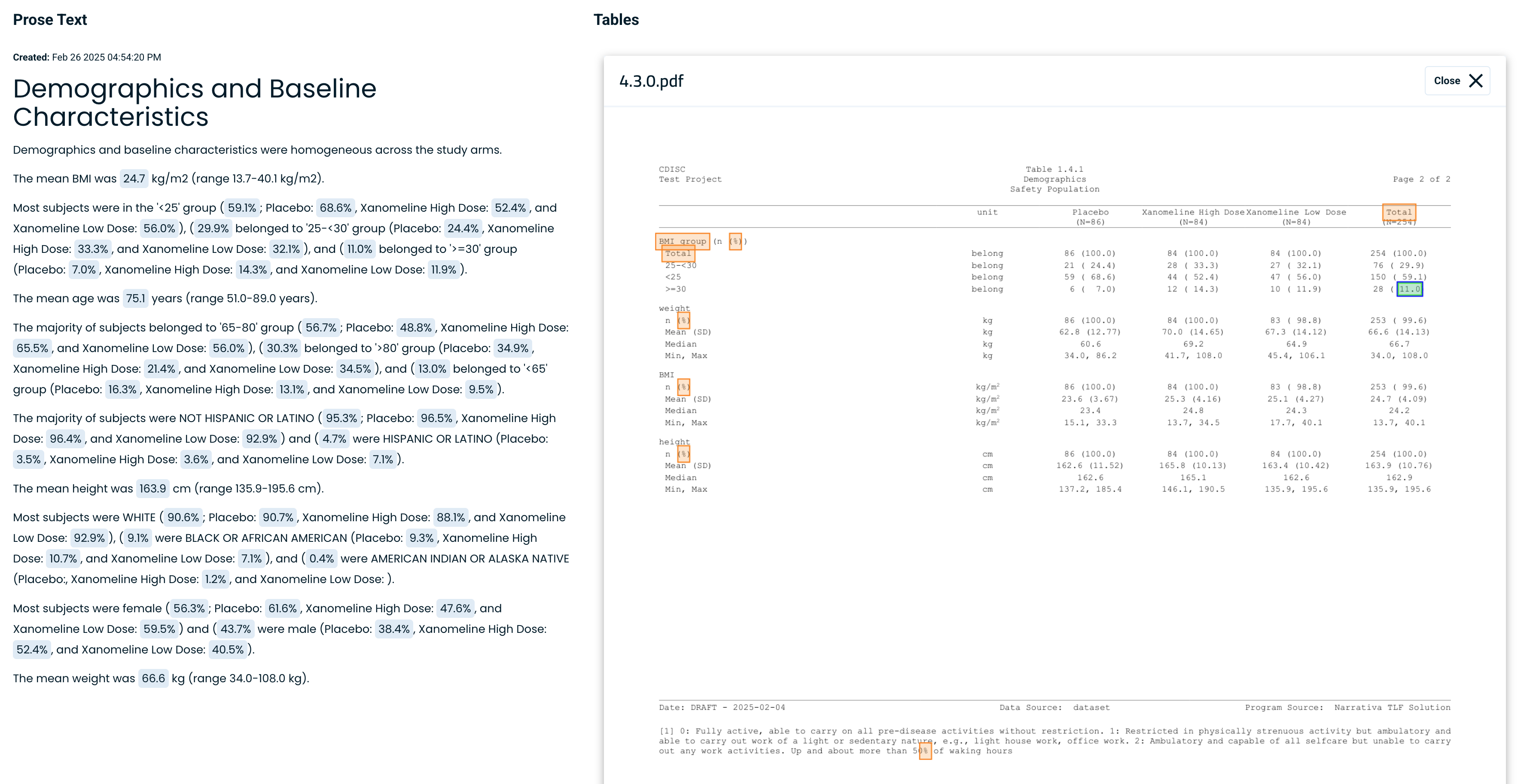
By clicking on any data point, the system will locate the corresponding data point in the appropriate table
Narrativa Knowledge Graph® integrates various data management methodologies, including:
- Interlinked Databases: Providing structured entity descriptions.
- Semantic Knowledge Bases: Enabling data interpretation and inference.
By combining deep learning with knowledge graphs, Narrativa can process vast datasets, extract key insights, and generate contextualized reports.
Advanced Statistical and Analytical Integration
Narrativa’s CSR Automation solution incorporates:
- Statistical testing for hypothesis validation
- Confidence interval calculations
- Risk assessments (relative risk, risk ratios)
- Frequentist and data augmentation techniques
More Features: Variability and Rich Language
Narativa’s CSR Automation Solution is specifically engineered to create quality content in a simple and effortless way. We require a corpus to train our system and adapt it to the appropriate or desired style so that; the final result will have variability and rich language if needed.
We use different deep learning algorithms to extract all the valuable information and ingest large amounts of medical trial data to generate the text. To achieve this in a more accurate way, we extract topics, synonyms, and similar sentences. The goal is to mimic the writing style used in the corpus.
In addition, companies maintain full control of the output. If the final result does not meet the expectations, text adjustments can be quickly made as the process is reiterated.
It is part of Narrativa’s mission to use technology for good, and transforming medical trial data into documentation is certainly a way that artificial intelligence can be democratized for the benefit of humanity.

CSR Generative AI Automation
The pharmaceutical industry is undergoing a data revolution, with AI playing a pivotal role in transforming raw data into actionable insights. Traditionally, empirical analysis has been the foundation for identifying patterns, testing hypotheses, and evaluating treatment efficacy. Now, AI is set to amplify this process, enabling faster knowledge dissemination and regulatory compliance.
Overcoming Regulatory Compliance Challenges
Regulatory compliance is a critical component of clinical trials, yet the manual creation of CSRs remains a resource-intensive task. Automating these reports allows pharmaceutical companies to:
- Optimize operational efficiency
- Reduce costs and manual workload
- Reallocate resources to higher-value research and development activities
By embracing AI-driven CSR automation, the pharmaceutical industry can expedite drug development, improve regulatory submissions, and ultimately enhance patient outcomes.
Book a demo to learn more about how Narrativa’s Generative AI Automation Platform can transform your business through Clinical Study Reporting Automation.
or Contact us




