January 28, 2024
Streamline the Authoring of eCTD Sections with Narrativa’s Generative AI platform

By Ehab Naim
The eCTD is a highly standardized submission format used by regulatory authorities. Creating its components comes with its own set of challenges, but automating sections of the eCTD documentation is possible today with the help of Narrativa’s Generative AI platform! Our platform helps automate the creation of key regulatory documents, such as CSRs, Protocol Narratives, and redaction processes, ensuring efficiency and compliance.
How Narrativa supports eCTD Documentation
- Automatically generate and populate sections and subsections of the eCTD, streamlining the documentation process.
- Integrate and reformat existing approved content for reuse in new submissions while ensuring regulatory consistency and compliance.
- Identify and redact any portion of reused text that no longer complies with the latest regulatory standards.
- Dynamically generate, cross-reference, and track changes between versions to ensure quality and a seamless review process.
- Unify formatting, style, and table structures across submissions for improved efficiency.
- Support your regulatory and medical writing teams, allowing them to focus on high-value tasks.
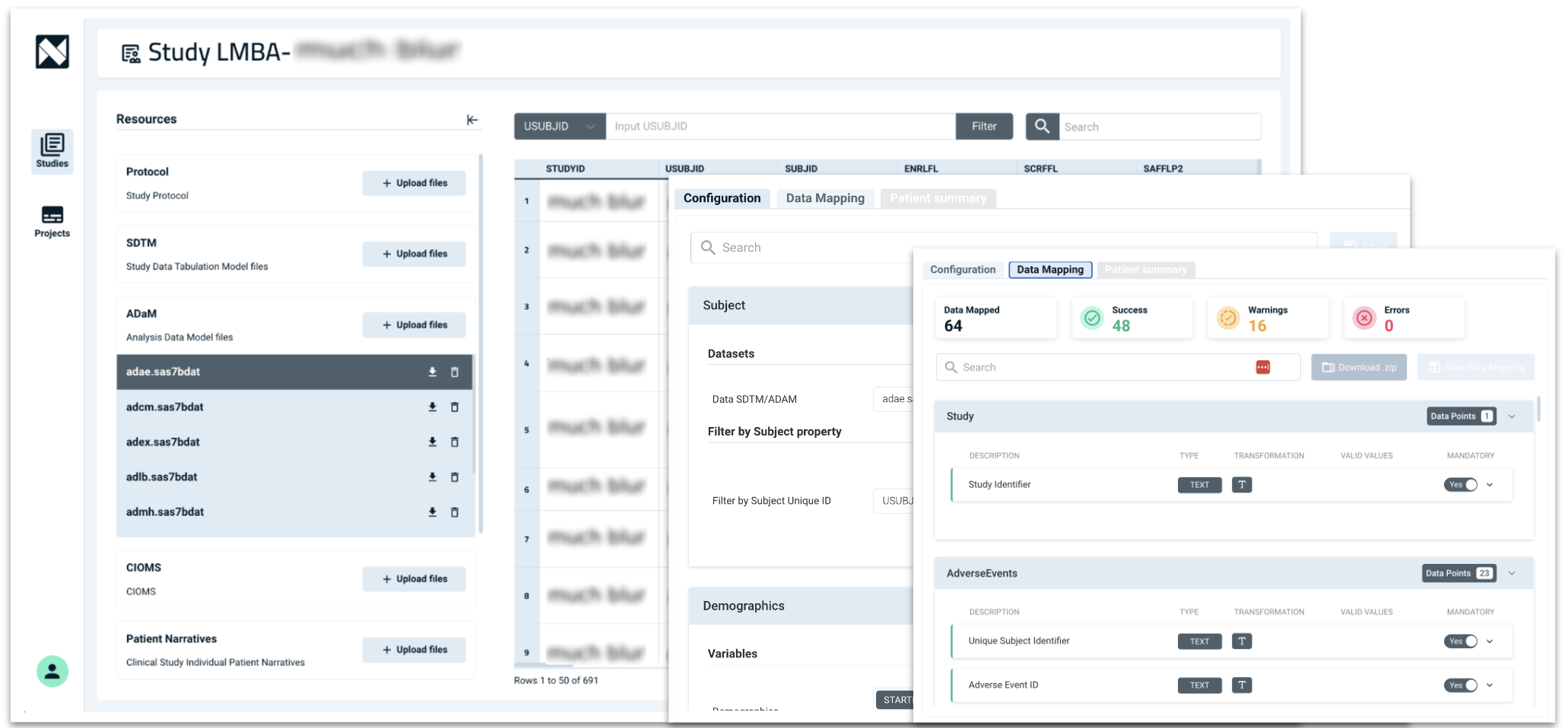
Our platform helps automate the creation of key regulatory documents
Background about the eCTD
The electronic Common Technical Document (eCTD) is a standardized format used by pharmaceutical companies to file regulatory submissions with authorities, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe.
This document is mainly comprised of five sections, including administrative information, Summaries, Quality, Nonclinical Study Reports, and Clinical Study Reports (CSRs). These sections contain various information about safety, efficacy, manufacturing requirements, label information, and other types of data needed to gain approval from regulatory agencies.
Challenges of Regulatory Compliance
Authoring and compiling the sections of this highly structured submission is a complex process, often taking several months and involving multiple stakeholders. Errors, inconsistencies, and evolving regulatory requirements can further complicate the process, leading to delays in review timelines and, ultimately, in delivering life-saving therapies to patients.
Moreover, variations in requirements between different health authorities, such as the FDA and EMA, combined with frequent updates and modifications directed by stakeholders, make compliance a moving target. Reusing approved content across multiple submissions can introduce inconsistencies if not properly managed, increasing the risk of non-compliance.
Be compliant and remain compliant with Narrativa
Narrativa overcomes these challenges with its Generative AI and automation technologies. Our platform does not completely automate the submission process but significantly enhances the efficiency of eCTD documentation preparation, ensuring compliance while reducing time and costs.
With Narrativa’s Generative AI platform, you can:
- Expedite the creation of eCTD sections, reducing manual effort and time spent on documentation.
- Complete months’ worth of work in just a few hours with automated drafting and formatting tools.
- Ensure data privacy and security, as your information remains within your cloud environment.
- Generate initial drafts of eCTD sections and subsections quickly, enabling faster review cycles.
- Maintain consistency, reliability, and regulatory accuracy across all documentation.
- Support medical writers, statistical programmers, biostatisticians, and other professionals involved in regulatory documentation and review.
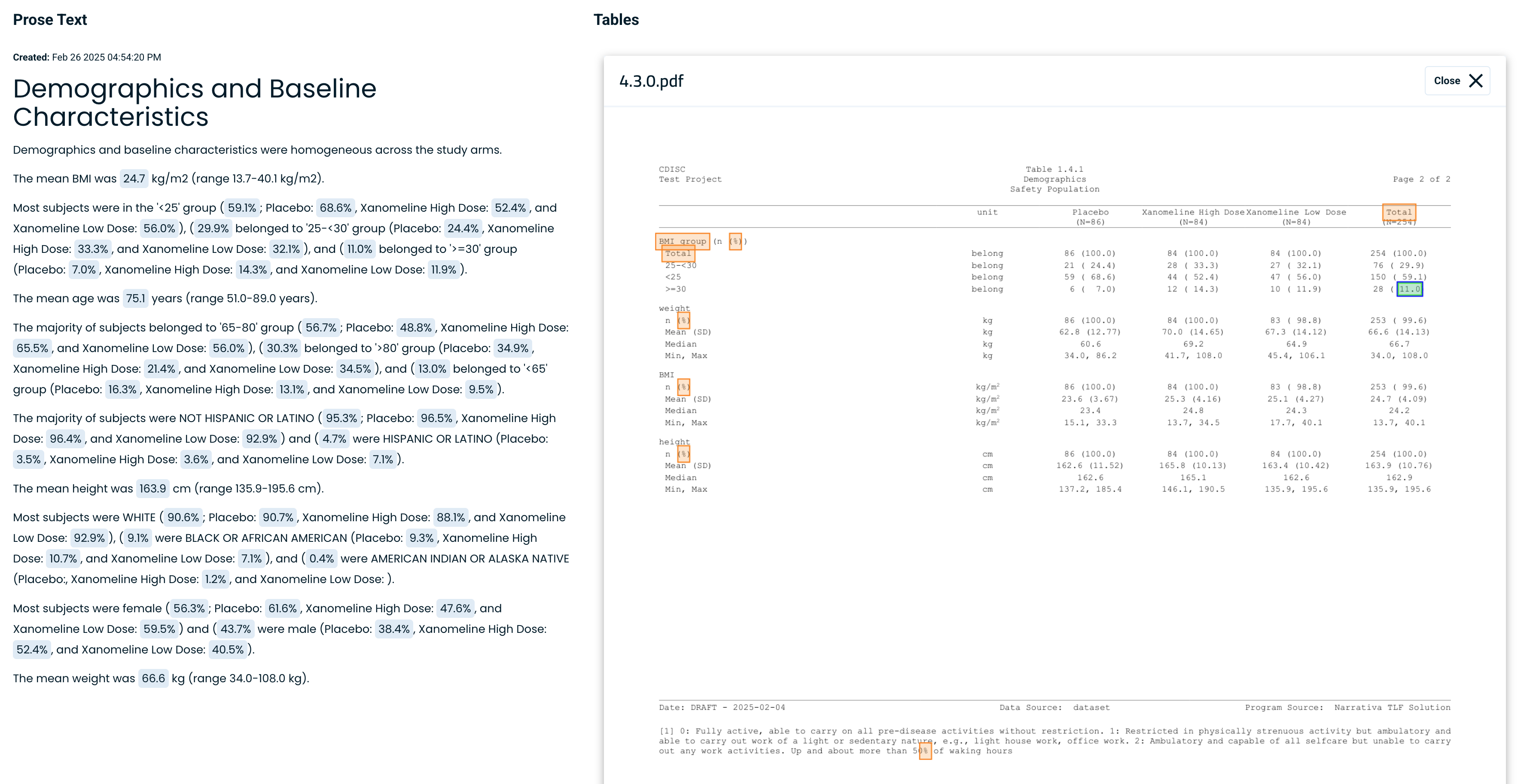
Maintain consistency, reliability, and regulatory accuracy across all documentation by clicking on any data point to find the source
At Narrativa, we use the latest generative AI and automation technologies to help you save time, money, and effort—while empowering your team to focus on critical processes that require human expertise. Improve the quality and consistency of your eCTD documentation today with Narrativa!
About Narrativa
Narrativa® is the leading generative AI automation company focused on revolutionizing the Life Sciences field. Its proprietary and secure medical writing automation platform, equipped with built-in AI agents, quickly and accurately creates smart documentation such as CSRs (clinical study reports), patient narratives, TLFs (tables, listings, and figures), and redacted/anonymized files that empower pharmaceutical sponsors, biotechnology companies, and CROs (clinical research organizations). From database to delivery, Narrativa not only speeds up the clinical trial process and reduces errors, but authors complete documentation for medical writer review and submission to regulatory bodies—all without the need for teams to piece together parts of documents themselves. Accelerate the potential with Narrativa®.
For additional information, visit www.narrativa.com and follow on LinkedIn, Facebook, Instagram, and X.
Share







