Regulatory Submissions Automation
Medical Writing Automation
Natural Language Generation
for Life Sciences
Narrativa’s Generative AI Automation Platform helps life sciences companies with streamlining the challenging and time-intensive processes associated with regulatory affairs.
Trusted by





Regulatory Submissions Automation
Medical Writing Automation
Narrativa’s Generative AI Automation Platform helps life sciences companies with streamlining the challenging and time-intensive processes associated with regulatory affairs.
Trusted by




1.5B USD
Yearly spend on regulatory documentation
40%
Expected cost reduction on medical writing
65%
Reduction on time spent on documentation generation
Artificial Intelligence as a Tool to Automate Regulatory Submissions
Regulatory submissions involve interaction between different departments to provide documents and files for medical writers to produce outcomes, like the clinical study report, patient safety narratives, TLFs and other documents. This process requires the medical writer to review thousands of inputs to make the final report. This could increase the chances of errors and problems. Generative AI in combination with an industry specific Knowledge Graph cut time and effort by automating the writing process so medical writers can focus on more critical processes.
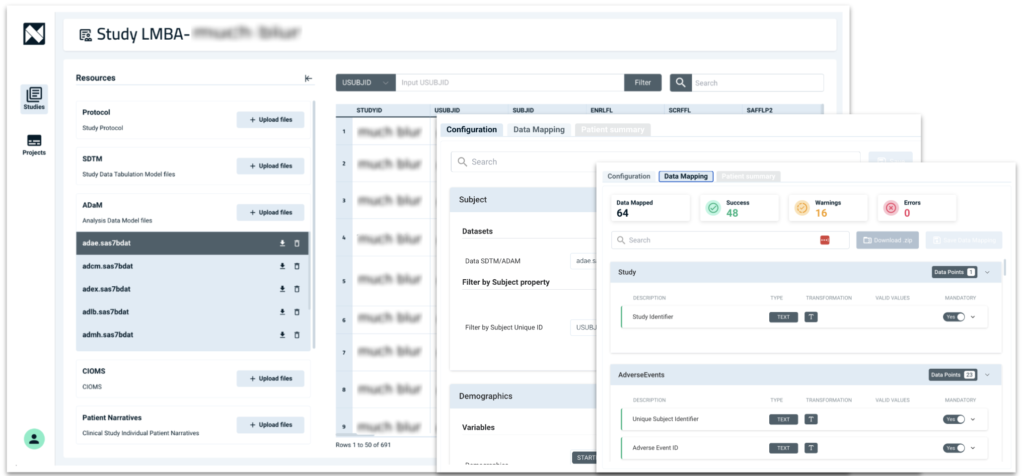
Improve Data Quality using AI
AI helps to enhance data quality by identifying potential issues early. The platform can detect anomalies, inconsistencies and flag missing data, ensuring higher data integrity and reducing the need for manual corrections. This proactive approach minimizes the risk of submission delays and improves the accuracy and completeness of the data used in reports.
By improving efficiency and data accuracy, AI helps medical writers and regulatory professionals ensure smoother, faster, and more reliable submissions.
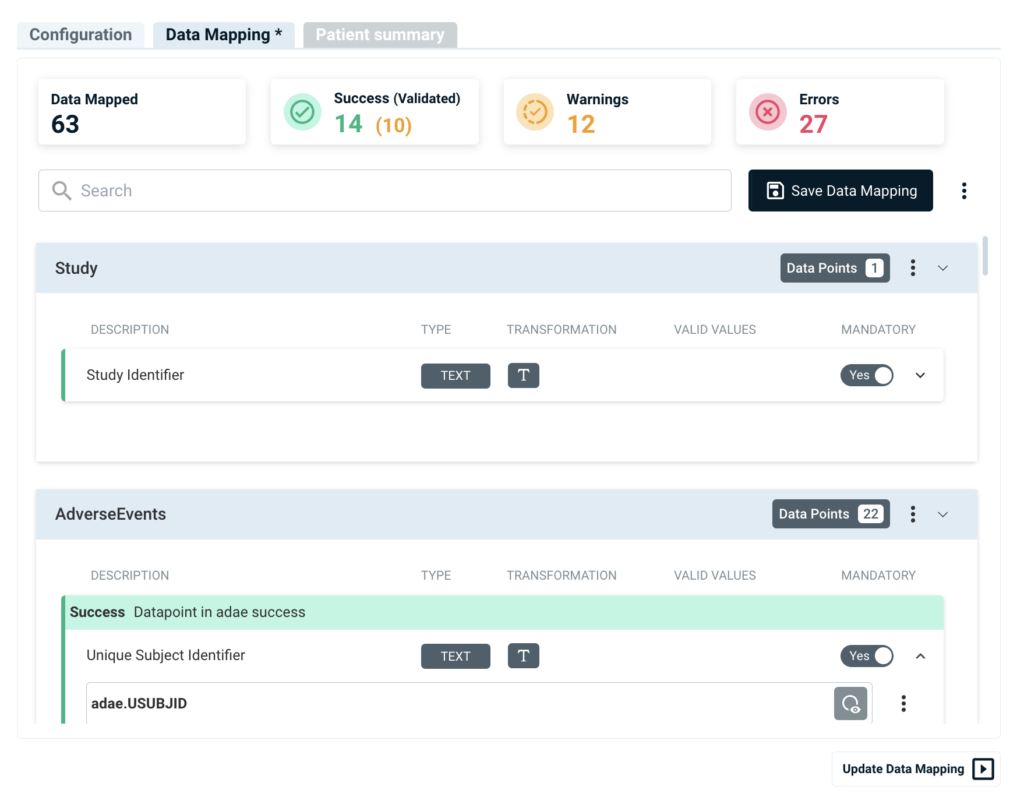
Life Sciences Insights

Medical Writing Generative AI Automation
Despite the utilization of AI in the healthcare industry, there are areas where the potential of this technology is underrepresented. Regulatory submission is one such area that could use AI and its subsets. Regulatory submissions, like clinical study reports, are based on information collected before, during, and after clinical trials. These data could come from many sources, including thousands of subjects across multiple regions and continents! It does not stop there. Someone needs to take these data, brush them up, and then put them in a form for others to go through them and generate the reports.
As mentioned above, regulatory submissions involve many parties. Still, Medical Writers (MWs) are the ones that are usually responsible for generating the reports needed to be submitted to health authorities, like the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This process takes a significant amount of time and effort to go through endless reports, tables, lists, and figures (TLFs) to create an outcome based on these inputs.
Generative AI is a technology that help an algorithm understand inputs, then generate an outcome that feels natural to the reader, as if a human wrote it. This technology can go through millions of inputs in a matter of seconds to minutes and generate entire reports. Often, these reports are 100% complete and require minimal supervision from the MWs. This way, MWs can focus on more critical processes requiring significant human input. The beauty Generative AI is that they can be made in a way that fits a template of your choosing, meaning that it is not a “one solution that fits all”. This ensures that you maintain your uniqueness and value.
Narrativa is a leader in Generative AI across multiple industries, including regulatory submissions. Many pharmaceutical, biotech, and clinical research organizations are riding the Digital Era wave. Be among the pioneers with Narrativa.
Book a demo to learn more about how our Generative AI Content Automation Platform can transform Life Sciences industry.